Your Global Automation Partner
Biotech and Pharmaceutical Industry: Short Time to Market through Modular Plant Construction
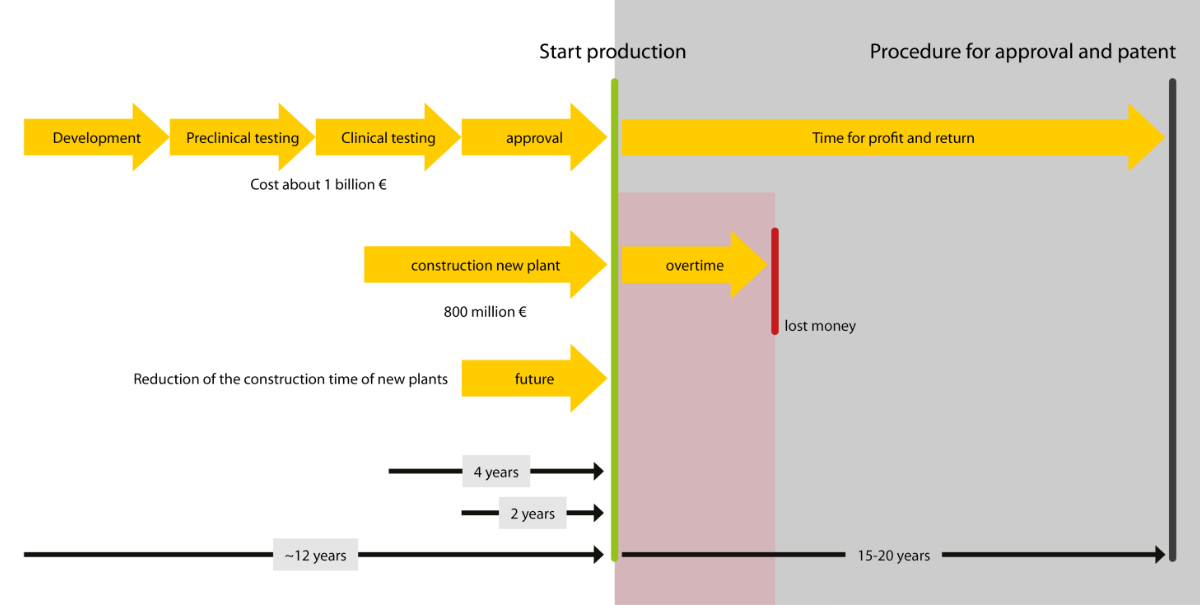
The growing demand for pharmaceutical products is presenting the biotechnology and pharmaceutical industry with some considerable challenges: the ability to react quickly to market requirements and make the time to market (TTM) for new plants as short as possible. But how can you speed up the development, production and installation of your modules? Turck has the answer: multiprotocol technology, which enables the modules of different manufacturers to be combined freely with the customer's control system. This means you only need a single gateway model – regardless of the customer.
In order to meet the growing demand for medical products, there is an increasing need for modular biopharmaceutical plants that can be set up quickly anywhere in the world. The use of tried and tested modules for biopharmaceutical plants with an identical design saves the effort required for plant engineering – and thus time and money. Modular plant construction in the pharmaceutical industry is benefiting from the increasing standardization of components.
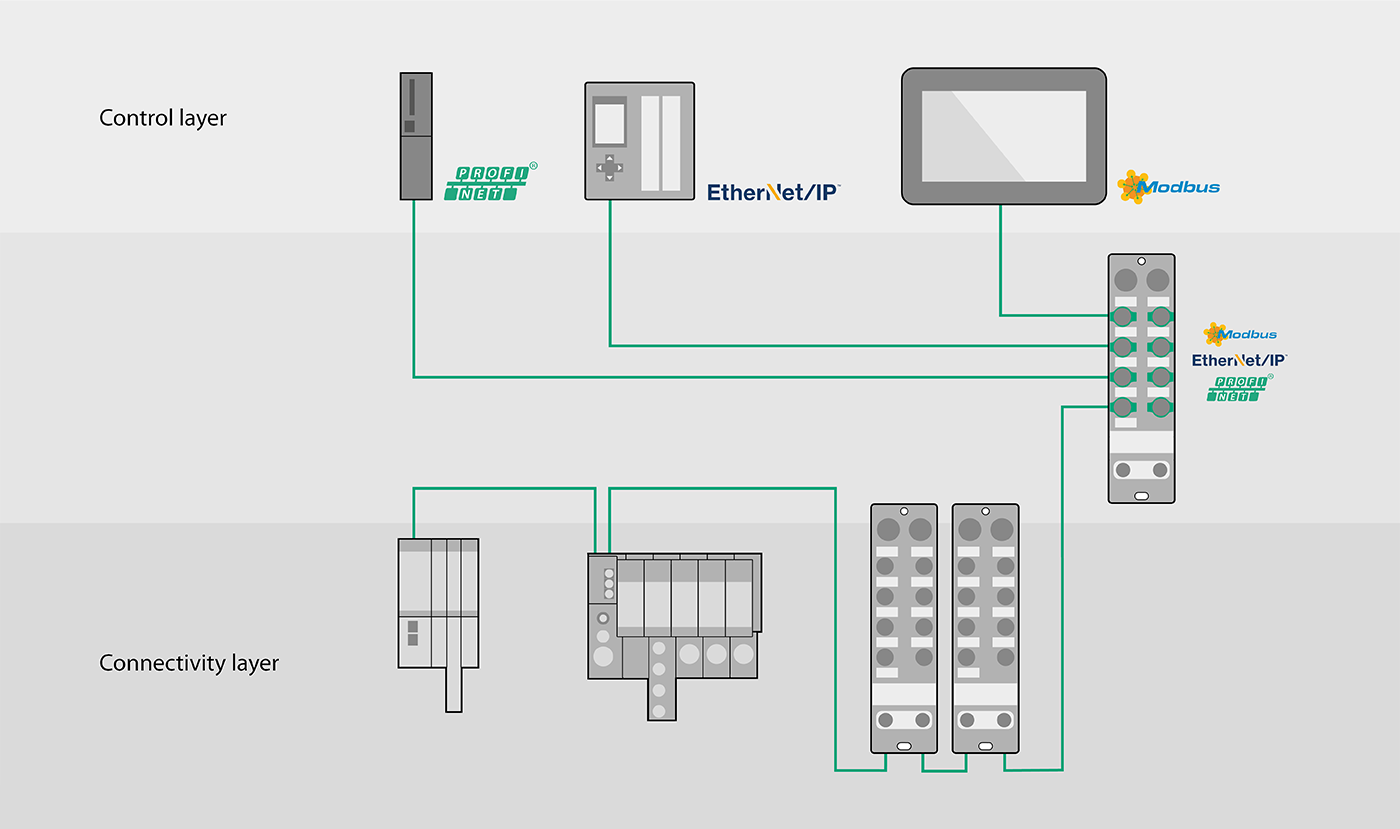
This is precisely where the benefits of Turck's multiprotocol I/O solutions for machine and plant builders come into their own. Your customers stick to their particular plant standards so that you have to develop and implement strategies for integrating the skids. Turcks' multiprotocol gateway enables your modules to operate in Profinet, Ethernet/IP and Modbus TCP networks without having to adapt the hardware. This means you only need a single gateway model – regardless of the customer. You benefit from shorter development and lead times as well as the reduced inventory costs. At the same time, you enable your customers to also have a considerably shorter time to market.

Cost reduction and shorter construction time
- Multiprotocol Ethernet reduces the number of device variants that have to be kept in stock
- No control cabinets needed thanks to decentralized IP67 modules

- Low inventory levels
- Reduced engineering effort
Fast commissioning
- Automatic identification of the connected modules via digital nameplates
- Module components already factory tested before commissioning
- FAT already at module manufacturer thanks to decentralized controller components

High availability
- Module diagnostic data via IO-Link
- Robust solutions, tried and tested on the market
- Constantly high quality with predictive maintenance
Webinar: Time to market in biotech and pharmaceutical production

Modular pharmaceutical plant construction in practice
Look at application examples of how users benefit from modular plant construction in the pharmaceutical industry – practical and instructive.
Products and solutions for the pharmaceutical industry:
I/O modules with multiprotocol Ethernet
All I/O modules with Turck multiprotocol Ethernet come as standard with some logic control functionality. The ARGEE programming user interface enables simple controller functions to be programmed on the module and executed by it – without the need to write program code or any additional costs.
IO-Link products
Turck's IO-Link portfolio boasts an outstanding degree of seamless integration. As a full supplier from the device to the master, Turck guarantees the easy integration of IO-Link devices in the controller environment.
RFID read/write heads and interfaces
Whether in the control cabinet or directly in the field as an IP67 complete system, RFID technology can decentralize decision making tasks and information, and thus help to increase flexibility and process safety.
Field Logic Controller (FLC, ARGEE)
Turck's FLC technology brings logic control to the field level. The ARGEE web-based programming environment adds logic functionality to Turck's block I/O modules with a multiprotocol Ethernet platform. This therefore turns I/O modules into field logic controllers (FLCs). Programming and configuration can be carried out without any software installation.
Reduce IP addresses with BEEP
Networks with up to 33 TBEN modules (1 master, 32 slaves) and up to 480 bytes of data can be connected to the PLC via a single IP address in Profinet, Ethernet/IP and Modbus TCP networks – thanks to Turck's Backplane Ethernet Extension Protocol or BEEP for short.

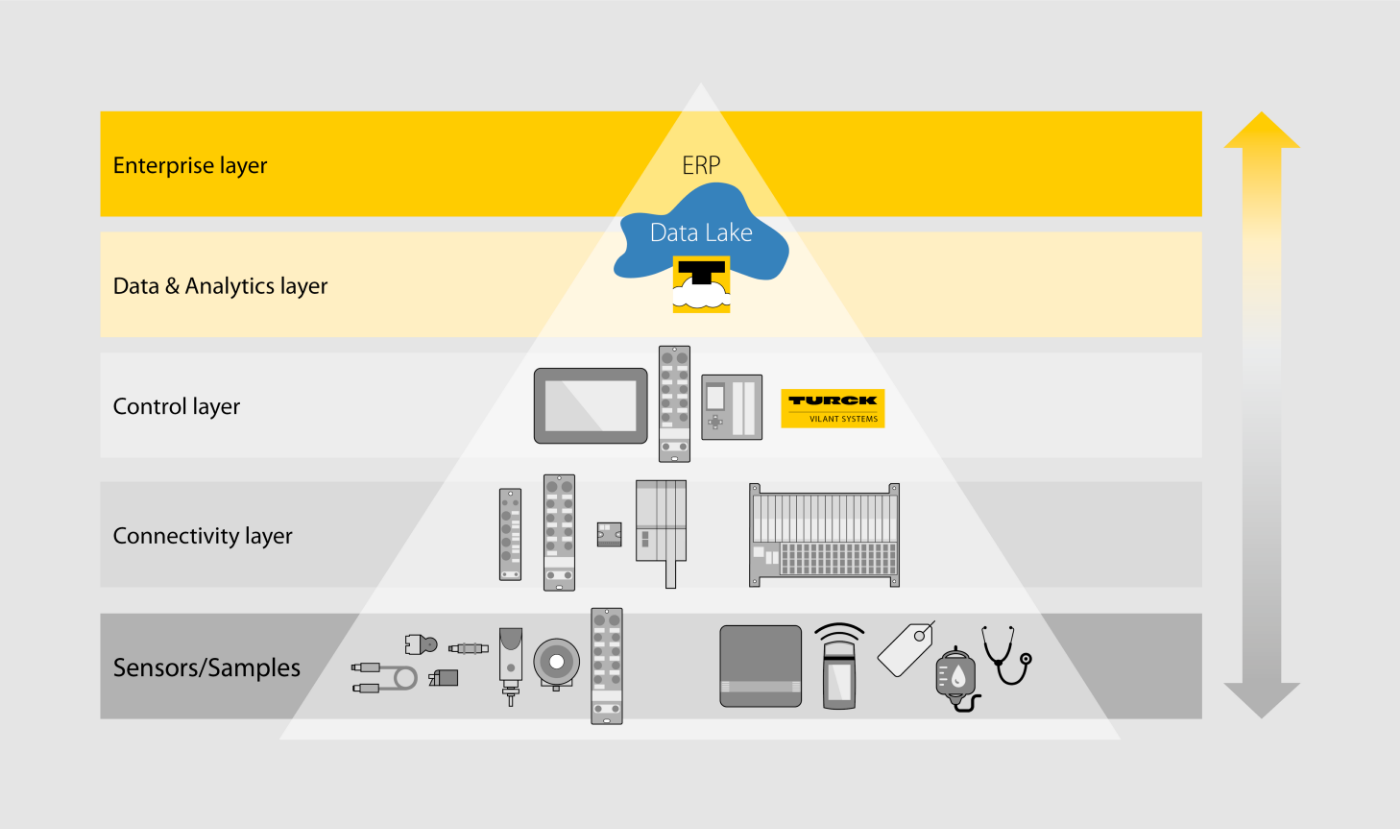
Turck Cloud Solutions
Optimized for industrial use, Turck's Cloud Solutions offer application specific services for internal plant transparency. Data can naturally also be stored in data lakes locally on your premises. Find out more about flexible monitoring and edge controllers with encrypted communication.
Ask an expert
Use or contact form if you have any questions on modular solutions or on a specific device. Our experts will be pleased to offer advice.


Dave Fazzini